As an at-risk sector, the life sciences industry has implemented layers of regulation to help reduce its susceptibility to fraud, bribery, corruption, privacy and other risks.
Significant Foreign Corrupt Practices Act (FCPA) enforcement activity and increased focus on UK Bribery Act compliance in recent years have acted as catalysts for reviews of legacy activity and the implementation of more robust anti-bribery and corruption practices for firms in the industry, particularly those with business interests in higher-risk geographic locations. Nevertheless, despite increased awareness, the life sciences industry continues to have high-profile issues causing significant financial and reputational impact.
The evolving environment in which a life sciences organization operates also leads to emerging risks, including increased susceptibility to cybercrime, counterfeiting, insider threat and adversarial social media activity.
How Can Kroll Help?
The product lifecycle diagram below highlights how Kroll has helped organizations within the life sciences industry address key risks.
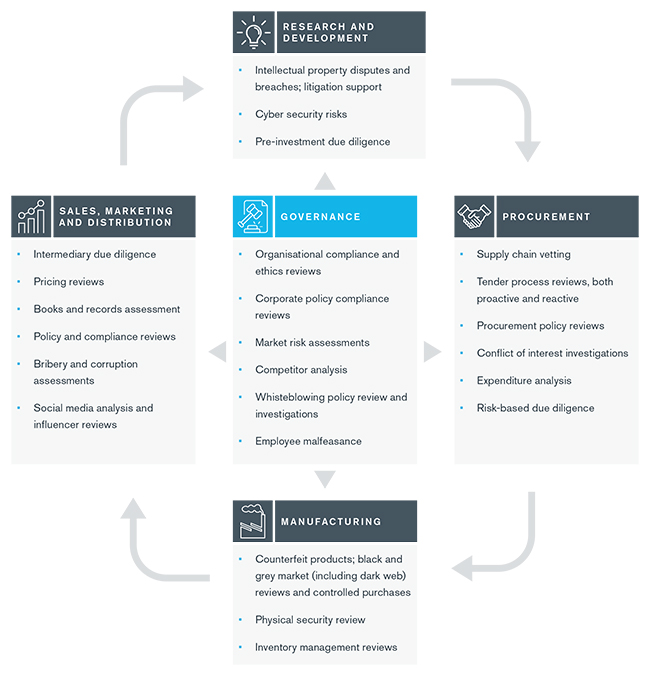
Return back to Healthcare, Pharmaceuticals and Biotech
Stay Ahead with Kroll
Forensic Investigations and Monitorships
The Kroll Investigations, Diligence and Compliance team consists of experts in forensic investigations and intelligence, delivering actionable data and insights that help clients worldwide make critical decisions and mitigate risk.
Cyber and Data Resilience
Kroll merges elite security and data risk expertise with frontline intelligence from thousands of incident response, regulatory compliance, financial crime and due diligence engagements to make our clients more cyber resilient.
Enterprise Security Risk Management
Kroll’s Enterprise Security Risk Management practice provides expert guidance and advisory services to our global clientele as they navigate the most challenging and emerging security and threat-related issues.